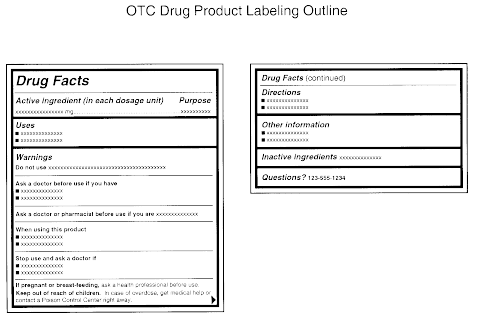
43 legal requirements for dispensing labels uk
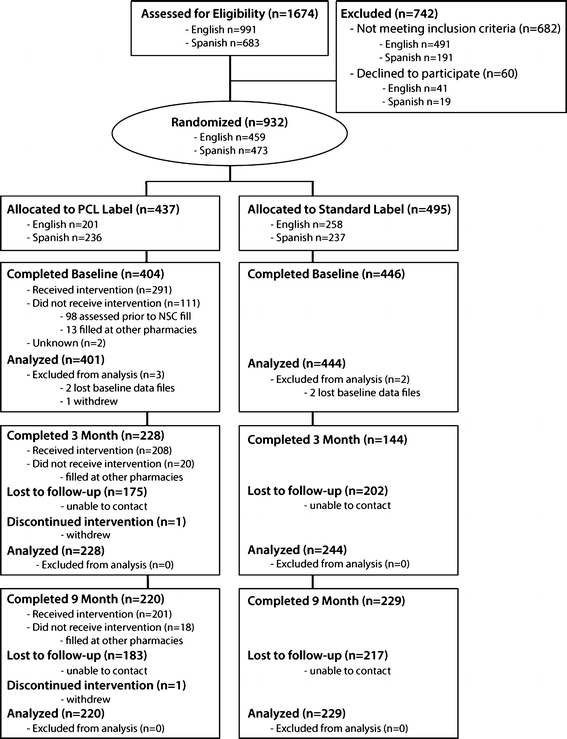
PDF BPGLPM tracked for EoT 041120 legal cleared - GOV.UK ,w pd\ eh qhfhvvdu\ lq vrph fdvhv wr h[suhvv wkh vwuhqjwk dv txdqwlw\ shu xqlw yroxph dqg dovr dv wkh wrwdo txdqwlw\ shu wrwdo yroxph 5hihuhqfh wr wkh wrwdo Labelling standards - Pharmacy Forum UK "apply 1-2 times a day" (bad practice to put numbers on labels also somebody with bad eyesight could see 12) "take two four to six hourly" (quite a few patients probably dont understand this) "take 1 3 times/day" "take ONE cap three times a day (ADVICE) after food" (use proper english!!!!!) "take two morning and night"
Food and Drink Labelling and Packaging Regulations 2021 - THE UK RULES The rules and regulations on food and drink labelling and packaging are strict in United Kingdom. Review how to label food packaging products and the legal requirements food businesses must follow. The main purpose of food labelling regulation is to help protect consumers. Thus, all product labels must provide accurate and correct information.

Legal requirements for dispensing labels uk
Optimising Dispensing Labels and Medicines Use The Human Medicines Regulations 2012 introduce changes to labelling and medicines-use which advance the clinical role of pharmacists in supporting people to get the most from prescribed medicines across the UK, providing greater clinical flexibility for prescription intervention. Drug storage and dispensing | BSAVA Library Oral liquids should be dispensed in plain glass bottles with child-resistant closures. All medicines should be labelled. The label should include: The owner's name and address Identification of the animal Date of supply (and, if applicable, the expiry date) Product name (and strength) Total quantity of the product supplied in the container Implementing the Falsified Medicines Directive ... - GOV.UK Oct 24, 2018 · The upper label is removed upon dispensing in Italy leaving a voided area and strip containing the repeated serial number. The complete Italian bollino label (2 layers) must be present on the ...
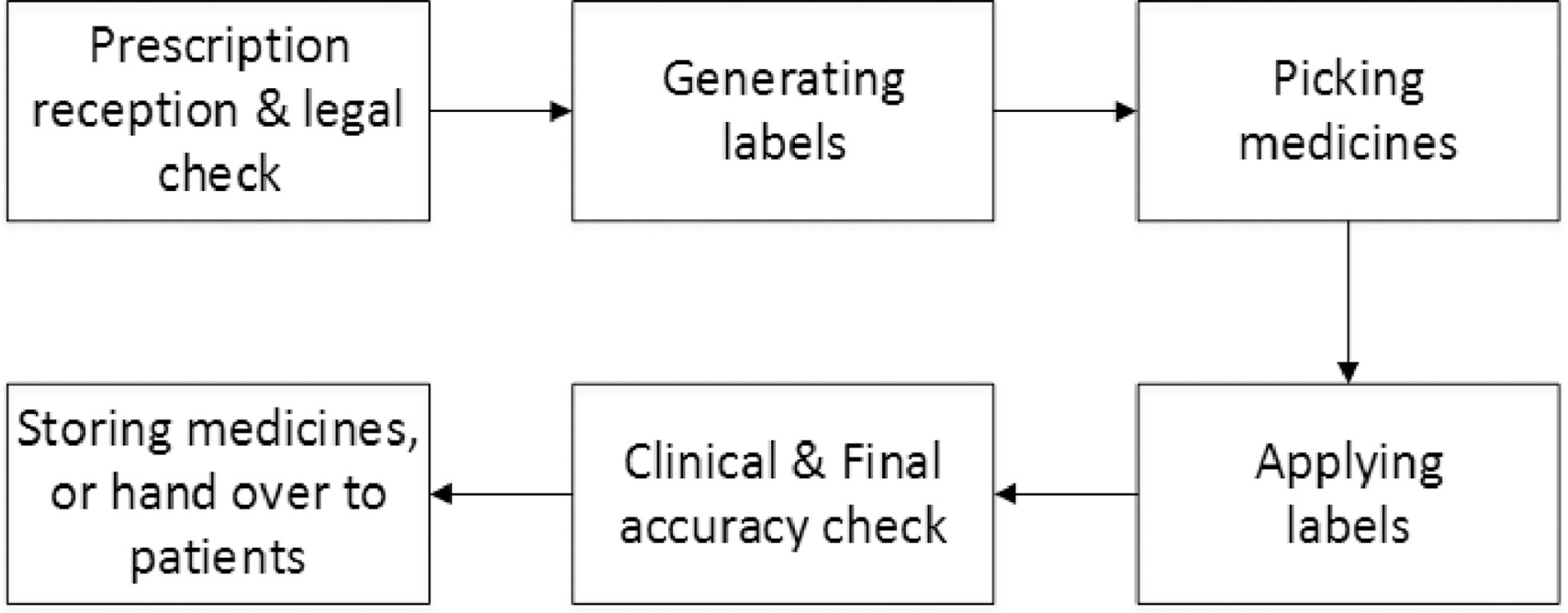
Legal requirements for dispensing labels uk. Candle Label Requirements - EU Edition • Armatage Candle Company What parts of the law you should care about as a candle maker; How Brexit impacts candle making legislation; How to design a compliant candle label in the UK and EU; As with all things about the law, do not consider this legal advice. Instead, treat this as a starting point for your own journey as a candle maker. Let's dive in! Prescription Dispensing - OCPInfo.com The resources below provide information for assessing the validity of a prescription, preparing prescriptions for dispensing and transferring or delivering prescriptions. Policies Cross-Jurisdictional Pharmacy Services Policy Distribution of Medication Samples Faxed Transmission of Prescriptions Fees for Professional Pharmacy Services Labelling Single Entity Drugs Guidelines Dispensing ... The Human Medicines Regulations 2012 - Legislation.gov.uk Sale and supply of starting materials. 33. Offence concerning data for advanced therapy medicinal products. 34. Offences: breach of regulations and false information and defence concerning starting materials. 35. Penalties. Conditions for holding a manufacturer's licence. 36. Dispensing Medicines - PSNC Website Pharmacies are required to maintain a record of all medicines dispensed, and also keep records of any interventions made which they judge to be significant. The Electronic Prescription Service (EPS) is also being implemented as part of the dispensing service. Service Specification
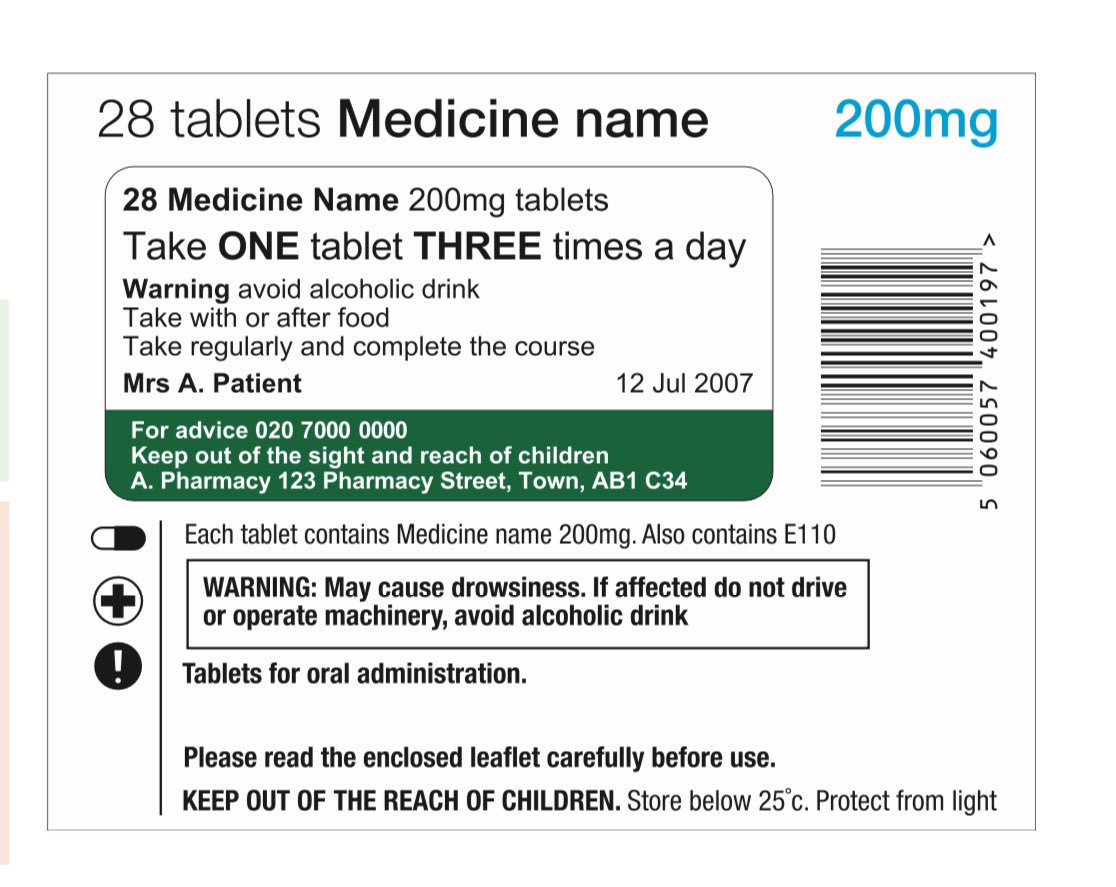
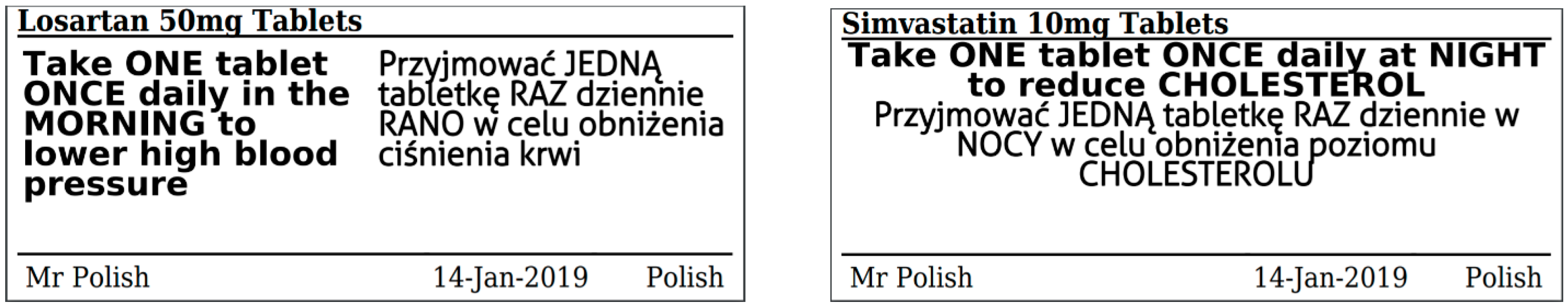
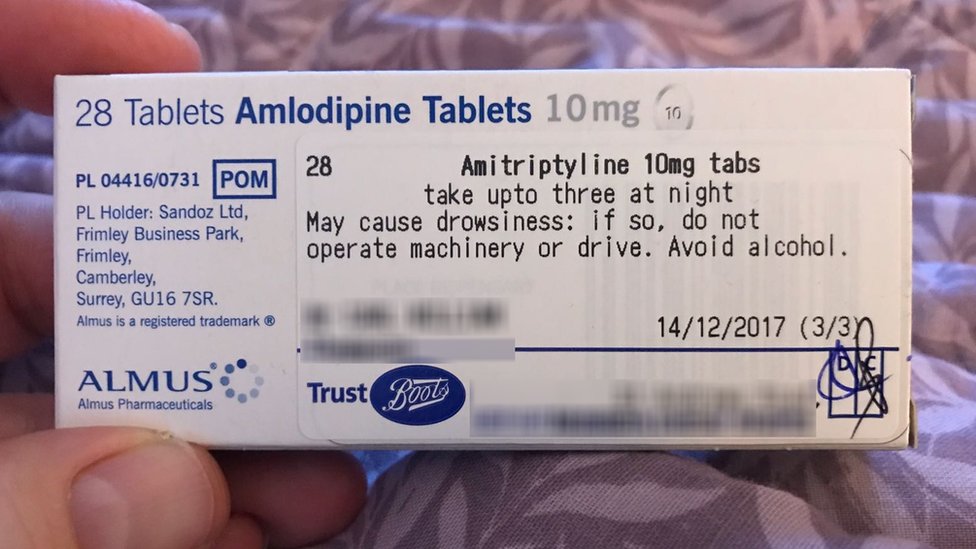
Medicines: packaging, labelling and patient information leaflets Labels must be clear. Healthcare professionals and patients must easily be able to identify the medicine by the label. You should use the letters CD in an inverted triangle if your product is a... Ireland - Labeling/Marking Requirements In Ireland, with only minor exceptions, there are no general requirements for marking imported goods with the country of origin. One notable exception is that the Irish authorities require that the name and the EU address of the manufacturer, distributor, or packer also appear on the label. Certain food products must show particulars of place ... Packaging & Labelling Requirements Guide | Handy Labels Requirements on the Primary Packaging On the primary packaging for cosmetics you will need to include: Contact details of responsible person or organisation Ingredient listings Batch code or number Shelf life Some means of identifying the function The average net weight or number of the product (s) Best before date Are Care Labels a Legal Requirement? - Business Services Week UK For such a small label, there are four areas of information that are required to be displayed: Fibre Content Mandatory for care labels in the UK, the fibre content of textiles must include information on the main fibre types used along with the percentages. The information must be easily understandable to the consumer, eg 80% wool, 20% cotton.
smol – here to help The aquatic life warning is an EU legal requirement for concentrated soaps and detergents. Our formulation is incredibly concentrated (which is why our capsules are so small!). They also contain very little water to minimise CO2 transport emissions. Their concentration puts them under specific EU label requirements. The Medicines (Labelling) Amendment Regulations 1992 - Legislation.gov.uk Special requirements for the labelling of the name of medicinal products for human use 4D. — (1) In any case where— (a) a relevant medicinal product is available in more than one pharmaceutical... Dispensing Requirements For Dispensing In Office - ProficientRX Nurse Practitioner or Physician Assistant credentials updated and legal if you plan to allow them to dispense medications. Your State issued dispensing license, if required, which it is in many States. In addition to meeting these dispensing requirements, many States require you meet the Pharmacy Board regulations also. FDA Issues New RX Label Requirements | RX Label Requirements for Opioids On July 1, 2019, the Food and Drug Administration issued new prescription label requirements . Medication that will be included under these new rules include those that are categorized under the Controlled Substances Act. Controlled substances are highly potent and potentially addictive medications, including opioids.
Packaging and labelling | Food Standards Agency How to display mandatory information on packaging and labels A minimum font size applies to mandatory information which you must print using a font with a minimum x-height of 1.2mm. If the...
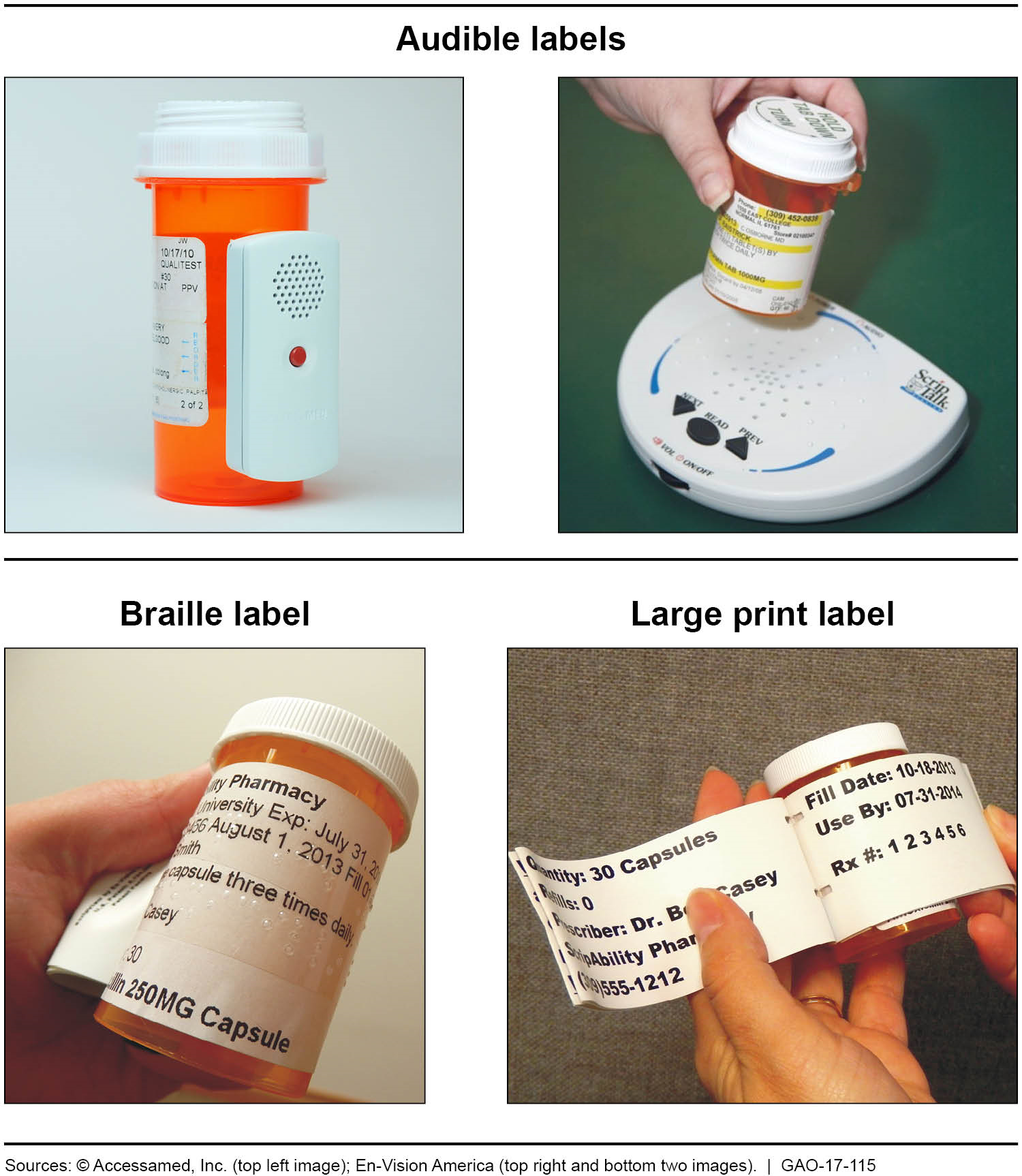
2 Labeling Prescriptions and Medications | Basicmedical Key C. Labeling and labels for dispensed drug products. 1. The NABP Model State Pharmacy Act defines the terms "label" and "labeling" for the purpose of pharmacist dispensing of drug products to patients as follows: a. Label: "A display of written, printed, or graphic matter upon the immediate container of any Drug or Device" ( 3 ).
Statutes & Constitution :View Statutes : Online Sunshine The dispensing organization must contract with an independent testing laboratory to perform audits on the dispensing organization’s standard operating procedures, testing records, and samples and provide the results to the department to confirm that the low-THC cannabis or medical cannabis meets the requirements of this section and that the ...
Rules & Regulations for Legally Selling Products | The Hub If you are making and selling products then you are the person that is held responsible for them. You will need to meet all the obligations set out in the regulations. It is your responsibility to ensure you are complying with the regulations. As well as carrying out all the necessary record keeping and safety precautions.
Dispensing a prescription - PSNC Website EPS Dispensing. EPS Submission. EPS Tokens. EPS Prescription Tracker. eRD. Medicines Database (dm+d) RTEC Exemption Checking. Records, Data Security & IG. Back to Records, Data Security & IG.
PDF Amendments to the Human Medicines Regulations 2012: 'hub and spoke ... • Clarify the dispensing label requirements of the Human Medicines Regulations 2012, in particular by updating the labelling requirements for monitored dosage systems to reflect current practice and by ensuring products supplied under patient group directions have a dispensing label in line with professional guidance; and
Microsoft is building an Xbox mobile gaming store to take on ... Oct 19, 2022 · Microsoft’s Activision Blizzard deal is key to the company’s mobile gaming efforts. Microsoft is quietly building a mobile Xbox store that will rely on Activision and King games.
Buy Delta 8 Gummies - Made With Premium Delta 8 THC Oil Are Delta 8 Gummies Legal? Our Delta 8 THC gummies are legal according to federal law and many state laws. Our Delta 8 THC distillate is 100% derived from legal hemp and does not contain more than 0.3% ∆9THC or any CBD. All Delta 8 products are under 0.3% delta 9 are federally legal.
Controlling Occupational Exposure to Hazardous Drugs Apr 04, 2011 · Note that the requirements of the HCS are superseded by those of OSHA's Laboratory Standard, 29 CFR 1910.1450, when an employer is engaged in the "laboratory use of hazardous chemicals" (i.e., use of relatively small quantities of hazardous chemicals on a non-production basis), but this document focuses on the HCS requirements that apply to ...
Labels and the Law (UK & EU) In the EU and the UK, manufacturers are legally required to state what textiles a garment is made out of. You must give an exact percentage of any material that comprises more than 15% of the total weight of the product, and every material must be listed on the label.
Labelling and packaging - Chemical classification - HSE Labelling and packaging. Labels are there to help identify hazardous chemicals and explain what the hazards are and how to avoid them. Packaging is also important to ensure that chemicals are stored and disposed of safely. Telling others about the classification: the hazard label. Hazard statements, precautionary statements and signal words.
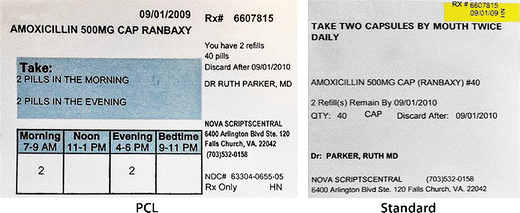
Prescription writing | Medicines guidance | BNF | NICE The age and the date of birth of the patient should preferably be stated, and it is a legal requirement in the case of prescription-only medicines to state the age for children under 12 years. These recommendations are acceptable for prescription-only medicines. Prescriptions for controlled drugs have additional legal requirements.
United Kingdom - Labeling/Marking Requirements UKCA markings must only be placed on a product by the manufacturer or an authorized representative. When affixing the UKCA marking, the manufacturer takes full responsibility for the product's conformity with the requirements of the relevant legislation. The UKCA marking must only be used to demonstrate conformity with the relevant UK legislation.
ERS Solutions Pharmacy Dispensing Labels | ERS Dispensing labels should be no smaller than 35mm x 70mm so that all printable information is readable, ensuring the correct medication in the correct dosage is administered to the correct patient. Pharmacy Label Options:
Best practice in the labelling and packaging of medicines Guidance Best practice in the labelling and packaging of medicines This guidance explains the legal framework for labelling and packaging as described in UK legislation and gives best...
Pharmacy Labels | PPL Leeds We create the laminate used to produce our dispensing labels so that we are able to assure quality of performance and give excellent value. ... United Kingdom T: +44 (0)113 213 4343 sales@ppl-leeds.co.uk. Twitter: ppl_leeds. Another week ahead of us! The sun is back - keep safe everyone! #covid19 #pplleeds 11:34:45 AM June 22, 2020 from Twitter ...
PPIC Statewide Survey: Californians and Their Government Oct 27, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and state legislators and to make ...
Legislation covering medicines | Department of Health Medicines legislation. The Human Medicines Regulations 2012 (SI 2012 /1916) which came into force on 14 August 2012, consolidate the law of the United Kingdom concerning medicinal products for human use ('products'). They set out a comprehensive regime for the authorisation of products; for the manufacture, import, distribution, sale and supply ...
Implementing the Falsified Medicines Directive ... - GOV.UK Oct 24, 2018 · The upper label is removed upon dispensing in Italy leaving a voided area and strip containing the repeated serial number. The complete Italian bollino label (2 layers) must be present on the ...
Drug storage and dispensing | BSAVA Library Oral liquids should be dispensed in plain glass bottles with child-resistant closures. All medicines should be labelled. The label should include: The owner's name and address Identification of the animal Date of supply (and, if applicable, the expiry date) Product name (and strength) Total quantity of the product supplied in the container
Optimising Dispensing Labels and Medicines Use The Human Medicines Regulations 2012 introduce changes to labelling and medicines-use which advance the clinical role of pharmacists in supporting people to get the most from prescribed medicines across the UK, providing greater clinical flexibility for prescription intervention.






















Post a Comment for "43 legal requirements for dispensing labels uk"